
Experience Structured Content AI at DIA RSIDM 2024
Experience the capabilities of generative AI-powered structured content authoring tools at DIA’s Regulatory Submissions, Information, and Document Management (RSIDM) Forum 2024.
Glemser will be on-site, showcasing our industry-tailored structured content AI software, ComplianceAuthor AI, and engaging in discussions on how regulatory and compliance teams are adapting workflows to align with the global transition to ePI and more consumer-friendly label content.
If you’re curious about AI-powered structured content or have questions for our team, fill out the form, and let’s meet at the show!




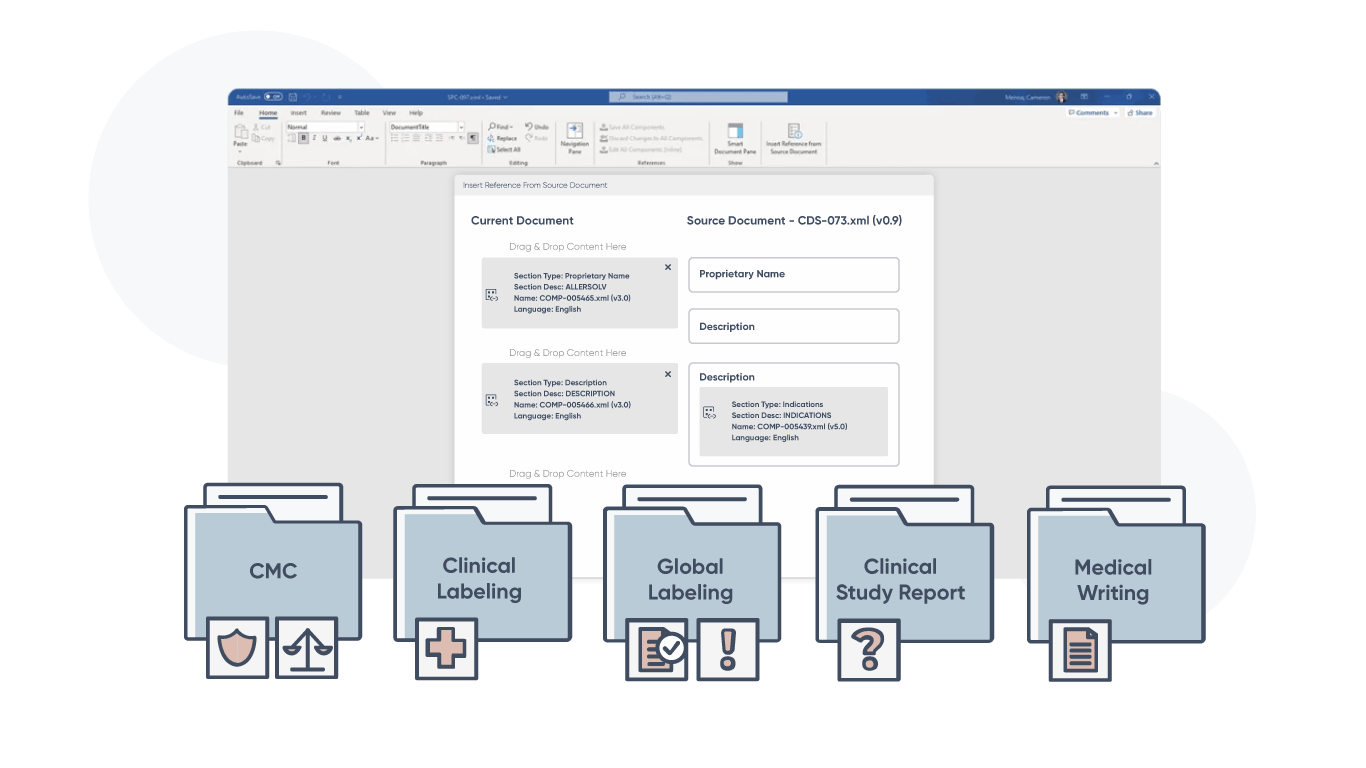
Component-based structured content. Simplified by AI.
Glemser helps pharmaceutical companies streamline the authoring and management of their most critical regulatory documents, efficiently and compliantly. Our ComplianceAuthor AI structured content AI platform empowers global teams to create, manage, and deploy structured (and unstructured) content across the enterprise. Automate your manual tasks, improve workflows, and generate submission-ready output in half the time.
DIA RSIDM Forum 2024
DIA’s Regulatory Submissions, Information, and Document Management Forum is tailored for regulatory professionals in pharmaceuticals and medical products. It provides a platform for collaborative discussions to enhance the development and management of accurate regulatory submissions and seamless exchange of information across regions and health authorities to ensure the safe and effective use of prescription drugs, biologics, and medical devices.
Bethesda North Marriott Hotel and Conference CenterFeb 12-14, 2024
5701 Marinelli Road
North Bethesda, MD 20852

© 2024 Connect Consulting all rights reserved