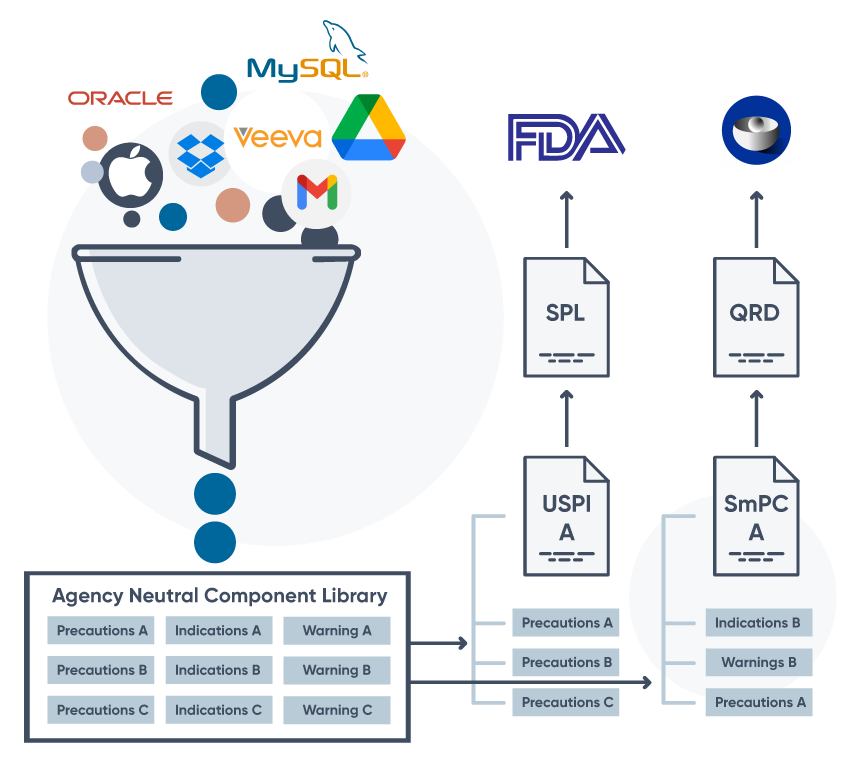
Streamline the way you draft and manage your most critical regulatory documentation with Glemser –structured content AI tools and IT solutions for pharma.
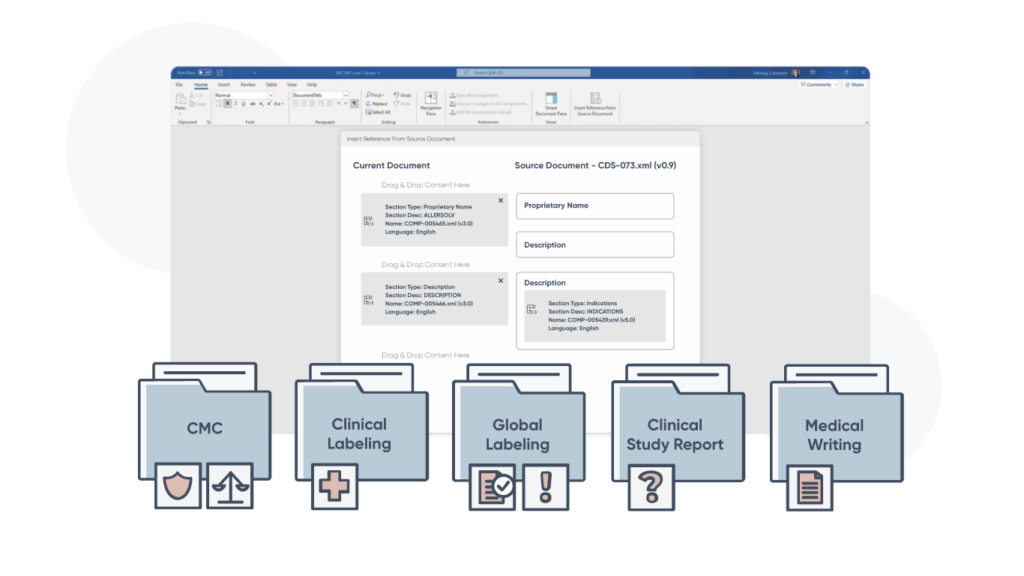


Our ComplianceAuthor AI® structured content platform empowers global teams to create, manage, and deploy structured content across the enterprise. Automate manual tasks, streamline workflows, and generate submission-ready output in half the time.
Leverage components to author and update faster, and then publish to many outputs.

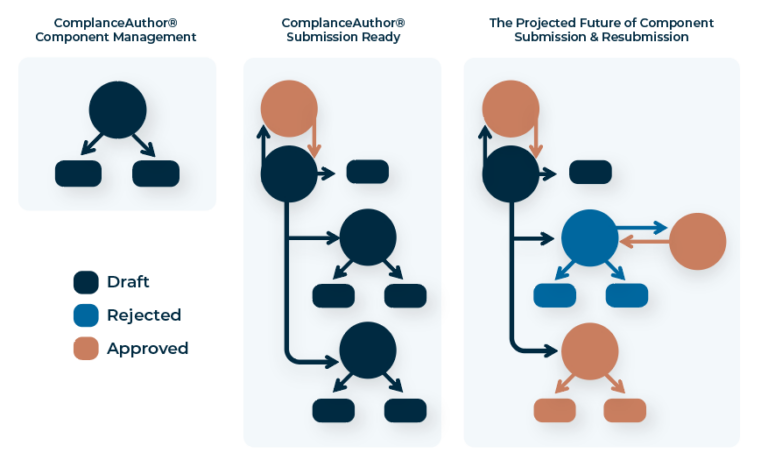
Create and deploy structured content components across the globe. Make a change once, and gain insights to determine the next best action.
Use AI pre-trained on life science content to instantly identify relevant documents, unlock insights, transform structured data, and author compliant outputs.
Apply a powerful, AI-powered toolset to use cases across global labeling, clinical labeling, test specifications for CMC, analytical procedures and more. And all without compromising compliance or safety.
Reduce the time to author and change product labels by 50%, from company core data sheets to local labels.
Allocate resources properly and reduce errors and risks to increase overall profitability.
Engineer superior, controlled components that sustain compliance and improve patient safety globally.
Translate content for your specific use case so it meets both language and regulatory requirements.
Our proven approach behind your firewall uses AI with a life sciences language model to automate the most cumbersome parts of your workflow, so your team is empowered to tackle operational challenges.
Enable automated data extraction and compare content against applicable regulations for an optimized workflow.
Enable automated data extraction and compare content against applicable regulations for an optimized workflow.
Enable automated data extraction and compare content against applicable regulations for an optimized workflow.