Centralize your global test, product, and procedure specifications—and analytical methods—in one AI-powered system that saves your team time, money, and effort supporting Module 3.


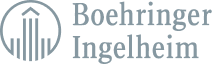


Easily manage and control the entire lifecycle for all your data-driven test specifications. All while maintaining a full audit trail and keeping compliance at the core of your products’ quality and safety.
ComplianceAuthor® AI ingests your test specifications and analytical methods allowing for global product quality and compliance in one central location.
ComplianceAuthor® AI allows users to quickly draft, deploy and link methods in a compliant manner, which eliminates redundant and duplicate documents.
ComplianceAuthor® AI helps users, health authorities and authorized third parties easily collaborate. Harmonize internal and external comments, edits, and tracked changes automatically in one document.
ComplianceAuthor® AI manages technical translations out of the box in support of your global operations. ComplianceAuthor AI supports both in-house and third-party translation service providers.
ComplianceAuthor® AI combines all various documents like test specification, methods, and validation reports to generate a specification report in a fully validated GxP system.
Centralize the creation and management of your CMC specifications in a single source of truth.
Reuse components, simplify authoring, and improve collaboration across teams.

Ensure accuracy and consistency by reusing analytical methods across test specifications.
From test methods to specifications and beyond, ComplianceAuthor AI® helps you organize CMC submission authoring and management across all your team’s functions and needs.
All substances and methods are housed in one system, ensuring compliance.
Improve and sustain compliance with drug products and methods in an integrated system.
Manage and re-use methods and tests within specifications.
ComplianceAuthor AI® ensures seamless team-based authoring and management for all your structured labeling content, dramatically reducing unnecessary rework and your reliance on manual workflows.