Reduce global product label generation time from months to days with an easy and cost-effective system your whole team will love.

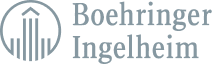


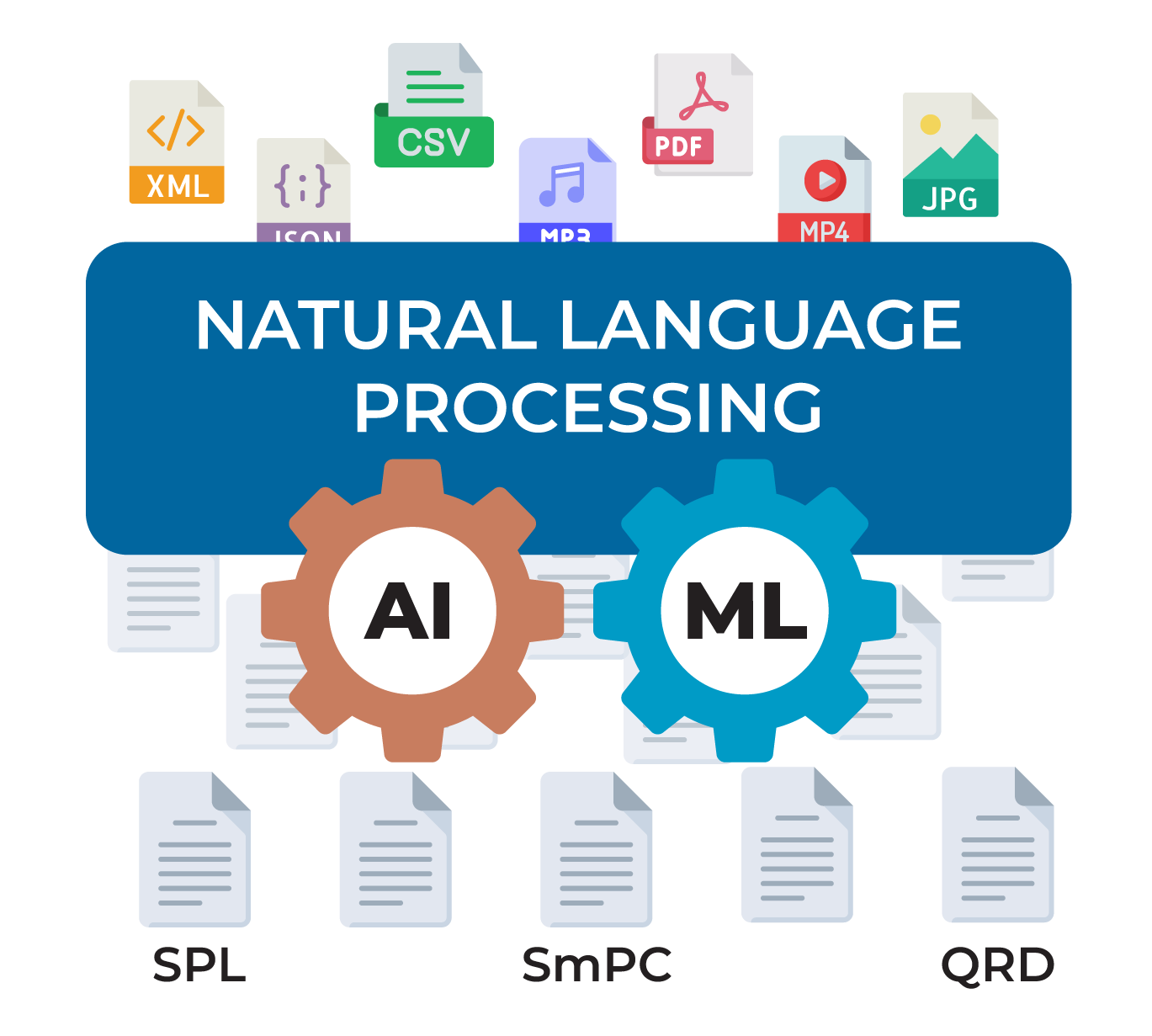
Create and deploy structured content components across the globe. Make a change once, and gain insights to determine the next best action.
Experience seamless compatibility with all health authority outputs, ensuring your labels are ready for every market.
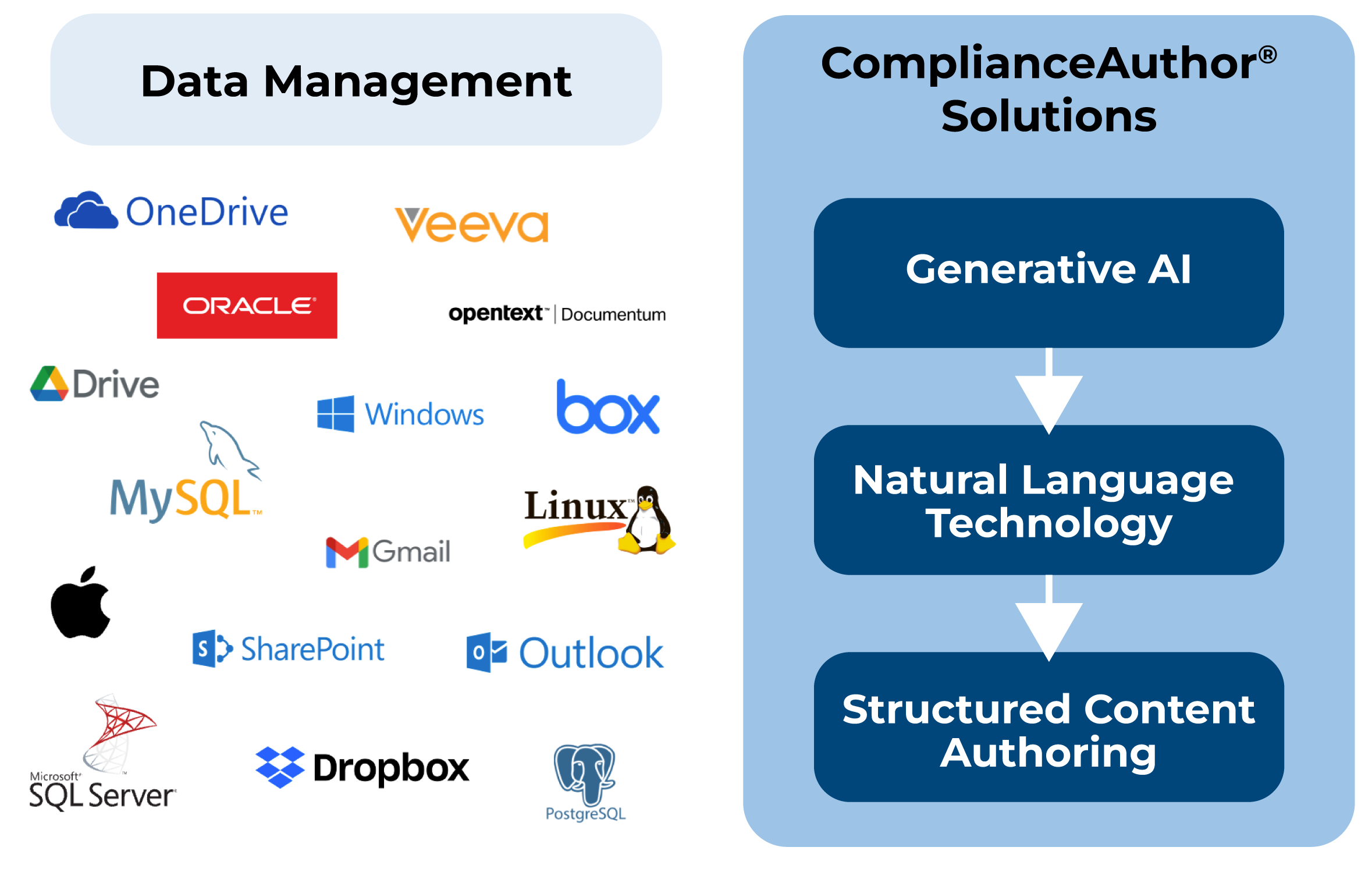
Search, find and index and monitor content. Manage change control across your enterprise, leveraging machine learning.
Design your product labels with regulated and compliant components at every stage of the drug’s lifecycle.
Use generative AI to transform internal data sheets into approved health authority labels, with a human in the loop.