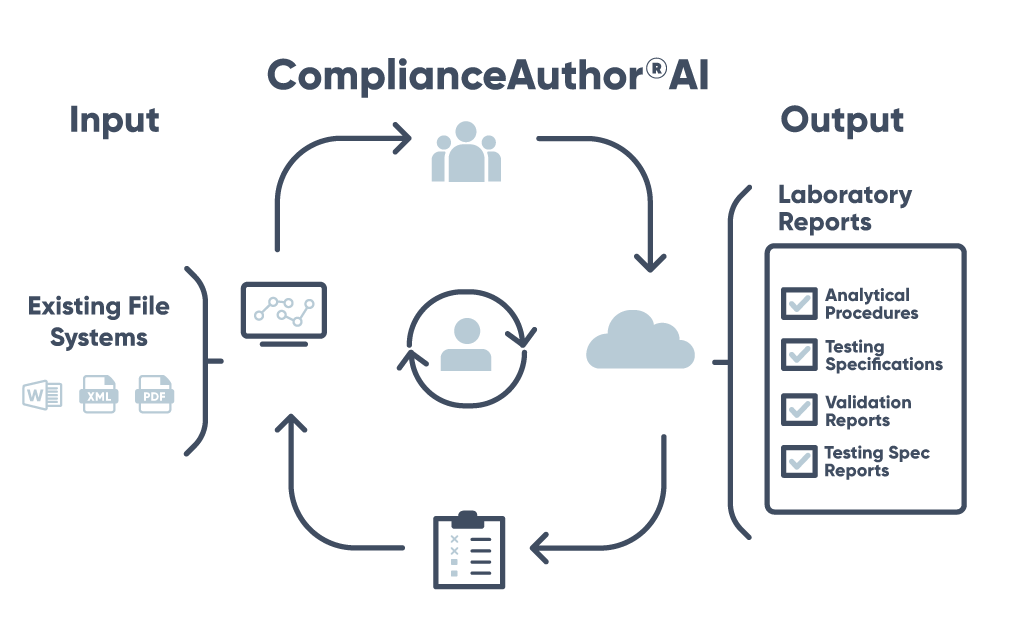
Centralize your Pharmaceutical Quality/Chemistry, Manufacturing, and Controls data—and analytical methods—in one AI-powered system that saves your team time, money, and effort.



Easily manage and control the entire lifecycle for all your PQ/CMC data. All while maintaining a full audit trail and keeping compliance at the core of your products’ safety.
ComplianceAuthor® AI allows users to quickly draft, deploy and link methods in a compliant manner, which eliminates redundant and duplicate documents.
ComplianceAuthor® AI helps users, health authorities and authorized third parties easily collaborate. Harmonize internal and external comments, edits, and tracked changes automatically in one document.
ComplianceAuthor® AI manages technical translations out of the box in support of your global operations. ComplianceAuthor AI supports both in-house and third-party translation service providers.
ComplianceAuthor® AI combines all various documents like test specification, methods, and validation reports to generate a specification report in a fully validated GxP system.
Centralize the creation and management of your PQ/CMC documents in one system.
Reuse components, simplify authoring, and improve collaboration across teams.

Ensure accuracy and consistency by reusing analytical methods across test specifications.
Centralize all raw material, in-process, and finished product data in a single system to maintain accuracy and compliance with global regulatory standards.
Improve and sustain PQ/CMC compliance with an integrated system that maintains consistency, traceability, and regulatory alignment.
Manage analytical procedures as controlled compliance components, ensuring accurate, audit-ready documentation for fast submissions.
PQ/CMC stands for Pharmaceutical Quality/Chemistry, Manufacturing, and Controls. It encompasses the entire process of ensuring the quality, safety, and consistency of pharmaceutical products through rigorous standards in product development, manufacturing, and regulatory compliance. For pharmaceutical labeling, PQ/CMC ensures that a product’s quality and safety data are accurately conveyed to regulators and end-users, meeting all necessary regulatory standards and ensuring consumer trust.
PQ/CMC ensures that all aspects of a pharmaceutical product, from raw materials and manufacturing processes to testing and packaging, comply with regulatory requirements. It provides a framework for collecting, documenting, and submitting data that meets the standards set by regulatory bodies such as the FDA, EMA, and other global regulators.
PQ/CMC ensures that pharmaceutical products are developed and manufactured according to the highest standards of quality and safety. PQ/CMC data, including chemistry, manufacturing, and control processes, must be submitted as part of a New Drug Application (NDA) or Biologics License Application (BLA) for approval. Proper PQ/CMC documentation provides regulators with the necessary evidence to evaluate the product’s safety, efficacy, and quality.
Structured content AI simplifies the creation and management of PQ/CMC documentation by automatically organizing, categorizing, and formatting regulatory content. This approach improves consistency, reduces manual errors, and ensures that the documentation is compliant with regulatory standards.
AI-powered solutions for PQ/CMC submissions automate time-consuming tasks such as data extraction, formatting, and validation, significantly reducing the risk of human error. They also improve the accuracy of submissions by ensuring that all necessary information is included and properly structured.
Automation eliminates many manual documentation processes that are prone to error, such as data entry, cross-referencing, and document formatting. It streamlines workflows by ensuring consistent, repeatable tasks are completed faster and more accurately.
Some of the common challenges in managing PQ/CMC documentation include maintaining document consistency, meeting compliance standards, handling large volumes of complex data, and coordinating cross-functional teams. Additionally, managing global submissions requires adhering to different regulatory requirements for each market, which can be time-consuming and resource-intensive.
PQ/CMC compliance modules are structured components of regulatory submissions that streamline document organization and ensure consistency. Examples include analytical procedures, test specifications, stability data, and manufacturing processes. These modules help maintain compliance by standardizing content, reducing errors, and improving efficiency across submissions.
Companies can stay ahead of changing PQ/CMC compliance expectations by closely monitoring regulatory updates from agencies such as the FDA, EMA, and ICH. Investing in AI-powered tools that automatically update and adapt to new regulations can ensure that documentation remains compliant.
HL7 FHIR (Fast Healthcare Interoperability Resources) ePI (electronic Product Information) streamlines the submission process by enabling the exchange of structured data between systems. By using standardized formats, FHIR ePI makes it easier to compile and share necessary documentation with global regulators, accelerating approval times and ensuring consistency across submissions in multiple regions.
HL7 FHIR ePI improves the quality of PQ/CMC data shared between systems by providing a standardized format for product information, making data more consistent, accurate, and interoperable. This reduces the likelihood of errors or discrepancies in the data, ensuring that regulators and stakeholders have access to up-to-date, high-quality information in real-time. Regulatory agencies like the FDA have begun efforts to accept HL7 FHIR structured PQ/CMC data as the future standard for electronic submissions.
ComplianceAuthor® AI offers several unique features, including the ability to automate document generation and published output, streamline the review process, and ensure compliance with global regulatory standards. Its AI-driven technology learns from your existing content to improve the accuracy and consistency of future submissions. Additionally, its ability to adapt to changing regulations and data standards like HL7 FHIR, as well as integrate with existing systems, makes it a highly flexible and efficient solution for managing PQ/CMC documentation.