Transforming Pharmaceutical Content Management with CCMS
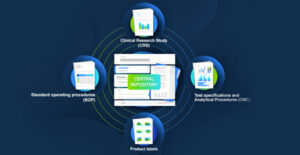
Component content management systems (CCMS) sit at the heart of an SCM strategy and enable content management at a more granular level. It meticulously organizes and manages content as components rather than documents, creating modular and reusable chunks of content that become interchangeable building blocks for various documents and submissions.